ACS Catalysis, 11, 608 – 624 (2021)
Catalytic Hydrogenation of Azobenzene in the Presence of a Cuboidal Mo3S4 Cluster via an Uncommon Sulfur-Based H2 Activation Mechanism
Eva Guillamón, Mónica Oliva, Juan Andrés, Rosa Llusar*, Elena Pedrajas, Vicent S. Safont, Andres G. Algarra*, and Manuel G. Basallote*
Abstract
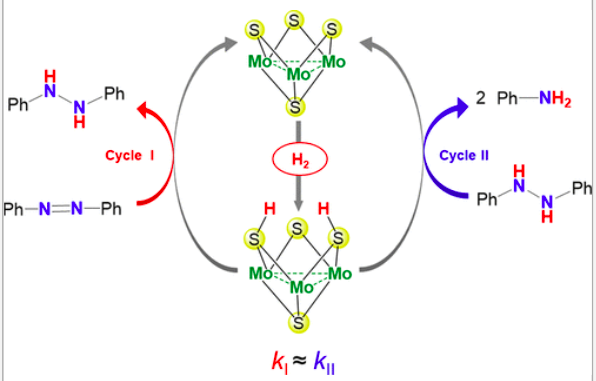
Azobenzene hydrogenation is catalyzed under moderate conditions by a cuboidal Mo3(μ3-S)(μ-S)3diamino complex via a cluster catalysis mechanism. Dihydrogen activation by the molecular [Mo3(μ3-S)(μ-S)3Cl3(dmen)3]+ cluster cation takes place at the μ-S bridging atoms without direct participation of the metals in clear contrast with classical concepts. The reaction occurs with the formation of 1,2-diphenylhydrazine as an intermediate with similar appearance and disappearance rate constants. On the basis of DFT calculations, a mechanism is proposed in which formation of 1,2-diphenylhydrazine and aniline occurs through two interconnected catalytic cycles that share a common reaction step that involves H2 addition to two of the bridging sulfur atoms of the catalyst to form a dithiolate Mo3(μ3-S)(μ-SH)2)(μ-S) adduct. Both catalytic cycles have similar activation barriers, in agreement with the experimental observation of close rate constant values. Microkinetic modeling of the process leads to computed concentration–time profiles in excellent agreement with the experimental ones providing additional support to the calculated reaction mechanism. Slight modifications on the experimental conditions of the catalytic protocol in combination with theoretical calculations discard a direct participation of the metal on the reaction mechanism. The effect of the ancillary ligands on the catalytic activity of the cluster fully agrees with the present mechanistic proposal. The results herein demonstrate the capability of molybdenum sulfide materials to activate hydrogen through an uncommon sulfur based mechanism opening attractive possibilities toward their applications as catalysts in other hydrogenation processes.
